IB Chemistry/Atomic Theory
Atomic Theory Revision Notes
edit2.1 The Nuclear Atom
2.1.1 : Protons and Neutrons form the nucleus of the atom, electrons orbit the nucleus in electron shells.
2.1.2 : Protons -- Mass = 1 amu , charge = +1 .. Neutrons -- Mass = 1 amu , charge = 0 .. Electron -- Mass = 1/1840 amu (usually insignificant), charge = -1
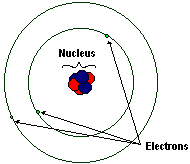
|
| A simple model of a lithium atom. Not to scale! |
Atoms are made up of a nucleus and electrons that orbit the nucleus.
The nucleus
editThe nucleus is made up of positively charged protons, and neutrons, which have no charge but about the same mass as a proton.
Electrons
editElectrons are negatively charged and move around the nucleus of an atom very quickly. So far, they do not appear to be made up of anything smaller: they are fundamental particles. They are extremely tiny, so small in fact that no one has managed to detect any size whatsoever. They are also very light, much much lighter than either a proton or a neutron. Hence, the weight of the electron is not included in the atomic number.
An atom in its natural, uncharged state has the same number of electrons as protons. If it gains or loses electrons, the atom acquires a charge and is then referred to as an ion. The number of protons in an atom defines its chemical identity (e.g. hydrogen, gold, argon, etc). Protons are not gained or lost through chemical reactions, but only through high energy nuclear processes.
2.1.3 : Mass number (A) -- Number of protons + neutrons. Atomic number (Z) -- number of proton. Isotope -- atoms with same atomic number but different mass number (i.e. different numbers of neutrons)
2.1.4 : XAz... A = mass number, Z = atomic number, X = atomic symbol.
2.1.5 : Isotopes may differ in physical properties (mass/density) and radioactivity but not generally in chemical properties.
2.1.6 : Atomic masses are the average of the atomic mass of each isotope (isotopic mass) times the isotope's relative abundance. results in non integer atomic masses
2.1.7 : Atomic number = number of protons (or number of electrons - ionic charge) , mass number - atomic number = number of neutrons.
2.2 Electron Arrangement
2.2.1 : Continuous spectrum goes continuously through red, orange, yellow, green, blue, indigo, violet. A line spectrum contains only some individual lines from this spectrum.
2.2.2 : Electrons are excited (usually by running an electric current through them). This causes electrons to 'jump' into higher electron shells ( X -> X* ) this state is only temporary, however, and the electron falls back to its ground state. This change (When the electron falls back from the higher shell to a lower one) decreases the energy of the electron, and this energy is emitted in the form of a photon. If this photon falls into the visible spectrum of light, then it produces a visible spectrum. As electrons move further away from the nucleus, the electron shells become closer together in terms of space and energy, and so lines converge towards the end of the spectrum.
Wave nature of electrons
editElectrons behave as particles but also as waves.
One of the results of this observation is that electrons can not orbit with any energy they like. Think of a standing wave on a guitar string. Only a whole number of half wavelengths will fit in the string to form a standing wave, likewise for an atomic shell. Since the energy is dependent on the wavelength this means that the energy of an electron in an atom (a bound electron) is quantized. This means that the energy is limited to certain distinct values, one for each shell with no middle values allowed.
2.2.3 : The main electron levels go : 2, 8, 18 etc...2n + 2 for n0, n1 and n2...
2.2.4 : Electrons are added from the left...after each shell is filled, move to the next...2, 8, 18...only up to Z = 20 is required.
HL Material
editTopic 12 is the additional HL material for Topic 2.
It's not just the energy that is quantized, other properties that an electron can posess are also split into distinct units with no in betweens. The angular momentum is quantised, the spin is quantised, the component of the angular moment in any direction that you care to choose is quantised. There are in fact a whole host of rules determining the values that each of these properties can take.
Electron shells
editEach different shell is subdivided into one or more orbitals, each of which has a different angular momentum. Each shell in an orbital has a characteristic shape, and are named by a letter. They are: s, p, d, and f.
In a one electron atom (e.g H, He+, Li++ etc.) the energy of each of the orbitals within a particular shell are all identical. However when there is more than one electron, they interact with one another and split the orbitals into slightly different energies. Within any particular shell, the energy of the orbitals depends on the angular momentum, with the s orbital having the lowest energy, then p, then d, etc.
The s Orbital
editThe simplest orbital in the atom is the 1s orbital. The 1s orbital is simply a sphere of electron density.
There is only one s orbital per shell. The s orbital can hold two electrons, as long as they have different spin quantum numbers.
The "P" Orbitals
editStylised image of all the 2p atomic orbitals.
Starting from the 2nd shell, there is a set of p orbitals. The angular momentum quantum number of the electrons confined to p orbitals is 1, so each orbital has one angular node. There are 3 choices for the magnetic quantum number, which indicates 3 differently orientated p orbitals. Finally, each orbital can accommodate two electrons (with opposite spins), giving the p orbitals a total capacity of 6 electrons.
The p orbitals all have two lobes of electron density pointing along each of the axes. Each one is symmetrical along its axis. The notation for the p orbitals indicate which axis it points down, i.e. px points along the x axis, py on the y axis and pz up and down the z axis. The p orbitals are degenerate, they all have the same energy. P orbitals are very often involved in bonding.
| 2px | 2py | 2pz |
|---|---|---|
|
|
The "D" Orbitals
editThe first set of d orbitals is the 3d set. There are 5 choices for the magnetic quantum number, which gives rise to 5 different d orbitals. Each orbital can hold two electrons (with opposite spins), giving the d orbitals a total capacity of 10 electrons.
Note that you are only required to know the shapes of s and p orbitals for the IB.
In most cases, the d orbitals are degenerate, but sometimes, they can split, with the eg and t2g subsets having different energy. Crystal Field Theory predicts and accounts for this. D orbitals are sometimes involved in bonding, especially in inorganic chemistry.
Material for new syllabus
editATOMIC STRUCTURE
editSL TOPIC 2.1 THE ATOM (1H).
editSEE NEUSS, P6-7 TOK: What is the significance of the model of the atom in the different areas of knowledge? Are the models and theories that scientists create accurate descriptions of the natural world, or are they primarily useful interpretations for prediction, explanation and control of the natural world?
The position of protons, neutrons and electrons in the atom.
editHere is a typical atom, helium:
TOK: None of these particles can be (or will be) directly observed. Which ways of knowing do we use to interpret indirect evidence gained through the use of technology? Do we believe or know of their existence?
the relative mass and relative charge of protons, electrons and neutrons.
editThe accepted values are:
| Relative Mass | Charge | |
| proton | 1 | +1 |
| neutron | 1 | 0 |
| electron | 1/1840 | -1 |
The mass of the atom is due to its nucleons (which is protons and neutrons).
An atom is electrically neutral because it has equal numbers of protons and electrons. electrons and protons.
Chemistry (basically every single chemical reaction) is solely because of the behavior of electrons.
The terms mass number (A), atomic number (Z) and isotopes of an element.
editThe atomic number of an atom is the number of protons.
The mass number of an atom is the number of nucleons (the number of protons + the number of neutrons).
The atomic number defines which element we are talking about. The element with 16 protons would be ‘sulfur’.
The symbols for isotopes
editThe following notation should be used AZX, for example 126C
Give the symbols for the following isotopes:
| Carbon-13 | Uranium-235 | Strontium-90 | Phosphorus-32 |
| 136C | 23592U | 9038Sr | 3215P |
The properties of the isotopes of an element.
editIsotopes have the same chemical properties but different physical properties.
‘Heavy water’ (21H2O or D2O) has the following properties:
Boiling point: 101.42 °C at standard pressure.
Freezing point: 3.81 °C at standard pressure.
Relative density: 1107 g dm-3 at STP.
Hydrogen-3 (‘Tritium’, T) is radioactive with a half-life of 12.32 years.
Carbon-14 is radioactive with a half-life of 5730 years.
CO2 normally has a density of 1.83 g dm-3 but if made with carbon-14 it would have a density of 1.92 g dm-3
The density of chlorine-35 gas is 2.92 g dm-3 under standard conditions, but chlorine-37 gas is 3.08 g dm-3.
The uses of radioisotopes
edit14C in radiocarbon dating
editLiving things constantly accumulate carbon-14 but the isotope decays with a half-life of 5730 y.
After death, accumulation stops but the decay continues, so the ratio of carbon-14 to carbon-12 can be used to calculate how long ago death occurred.
This can be used to date organic material from archaeological sites.
60Co in radiotherapy
editCobalt-60 emits gamma radiation which can be directed onto tumours in an attempt to kill their cancerous cells. Whole-body irradiation can be used to destroy bone marrow before a transplant is attempted.
131I and 125I as medical tracers
editThe thyroid is the only organ of the body which accumulates iodine, so isotopes of iodine can be used to study thyroid disorders. Iodine-131 or iodine-125 are given to patients in very low doses, and the pattern of radiation can reveal tumours or other abnormal growths. Larger doses of iodine radioisotopes can be used as therapy for thyroid cancer.
The number of protons, electrons and neutrons in atoms and ions
editComplete the following table:
| Symbol | Protons | Neutrons | Electrons |
|---|---|---|---|
| 115B | 5 | 6 | 5 |
| 6329Cu+2 | 29 | 34 | 27 |
| 20782Pb+2 | 82 | 125 | 80 |
| 3717Cl- | 17 | 20 | 18 |
| 19779Au+3 | 79 | 118 | 76 |
2.2 THE MASS SPECTROMETER
editThe operation of a mass spectrometer.
editSchematic diagram of a mass spectrometer.
How the mass spectrometer may be used to determine relative atomic mass
editBy varying the strength of the magnetic field, ions of different masses can be brought to focus on the detector. In this way the relative abundances of ions of different masses produced from the sample can be determined. This is known as a mass spectrum. Usually the electron bombardment is adjusted to produce ions with only a single charge. Any doubly charged ions will be deflected more than the singly charged ions and will in fact behave in the same way as a singly charged ion of half the mass. That is why the x-axis is labeled m/z, where m is the relative mass of the species and z its relative charge. For example, Sulfur-32 (2+) will be observed at m/z=16.
Calculation of non-integer relative atomic masses and abundance of isotopes
editThe relative atomic masses of many elements are not whole numbers. This is because they are mixtures of isotopes. Each isotope has a molar mass which is (almost) an integer e.g. the molar mass of chlorine-35 is 35.0 g mol-1 and chlorine-37 is 37.0 g mol-1.
Chlorine is a mixture of 24 % chlorine-37 and 76 % chlorine-35.
The molar mass is therefore: 37 x 0.24 + 35 x 0.76 = 35.48
Secret information: Carbon-12 is the only isotope with an exact integer for its molar mass. Other isotopes have molar masses which are almost, but not quite, whole numbers. The IB don’t require you to know this – but it explains why the values you calculate don’t always match the values in your data booklet.
This is the mass spectrum of mercury. There are two types of information we can find from this spectrum:
Mercury has six isotopes
Mercury-202 is the most common isotope
The molar mass of mercury is actually the average of the molar masses of its isotopes. From the table we can read these values:
| Isotope | Signal strength |
|---|---|
| 198 | 33.8 % |
| 199 | 56.4 % |
| 200 | 77.5 % |
| 201 | 44.3 % |
| 202 | 100 % |
| 204 | 23.0 % |
Note that ‘%’ does not mean ‘percent of the total mercury ions’. It means ‘percent of the most intense signal’. The total ‘percentage’ is 335 %.
To find the average, we treat ‘%’ as moles:
33.8 moles of mercury-198 have a mass of 6692.4 g
| Molar mass of isotope
(g mol-1) |
Amount (mol) | Mass (g) |
|---|---|---|
| 198 | 33.8 | 6692.4 |
| 199 | 56.4 | 11223.6 |
| 200 | 77.5 | 15500 |
| 201 | 44.3 | 8904.3 |
| 202 | 100 | 20200 |
| 204 | 23.0 | 4692 |
| total | 335.0 mol | 67212.3 g |
So we have a molar mass for the average mercury atom: = 200.6 g mol-1
Classwork 1. Copper has two stable isotopes:
Isotope Abundance (%) 63Cu 69.1 65Cu 30.9 Calculate the molar mass of copper.
2. Bromine has two isotopes, 79Br and 81Br. Look up the molar mass of bromine. What is the proportion of the two isotopes?
3. Boron has two isotopes, 10B and 11B. What is the proportion?
4. Lead has four stable isotopes:
Isotope Abundance (%) 204Pb 1.5 206Pb 23.6 207Pb 22.6 208Pb 52.3 Calculate the molar mass of lead.
1 An experiment was performed to determine the density of gold. The following measurements were recorded.
Mass of sample of gold = 30.923 g (to 5 sig fig),
Volume of sample of gold = 1.6 cm3 (to 2 sig fig).
Which of the following is the most accurate value for the density of gold (in g cm-3) which can be justified by these measurements?
A. 19.327 (to 5 sig fig)
B. 19.33 (to 4 sig fig)
C. 19.3 (to 3 sig fig)
D. 19 (to 2 sig fig)
2 The nucleus of a radon atom 22286Rn, contains A. 222 protons and 86 neutrons. B. 86 protons and 136 neutrons. C. 86 protons and 222 neutrons. D. 86 protons, 136 neutrons and 86 electrons.
3 Which of the following statements is/are true according to our current picture of the atom?
I More than 90 % of the mass of a given atom is found in its nucleus.
II Different atoms of an element may have different masses.
III The chemical properties of an element are due mainly to its electrons.
A. I only B. I and II only C. II and III only D. I, II and III
4. In which pair do the species contain the same number of neutrons? A. 10846Pd and 11048Cd B. 11850Sn and 12050Sn C. 19678Pt and 19878Pt+2 D. 22688Ra+2 and 22286Rn
5 Which pairing of electrons and protons could represent a Sr+2 ion? Protons Electrons A. 38 36 B. 38 38 C. 38 40 D. 40 38
6 All isotopes of tin have the same I. number of protons; II. number of neutrons; III. mass number. A. I only B. II only C. III only D. I and III only
2.3 ELECTRON ARRANGEMENT (2H)
editThe electromagnetic spectrum.
editThe electromagnetic spectrum unifies a vast range of ‘waves’, ‘rays’ and ‘radiation’.
All electromagnetic radiation travels at 299776 ms-1 in a vacuum.
Parts of the spectrum can be specified by their wavelength, frequency, or energy.
The electromagnetic spectrum. The red line indicates the room temperature thermal energy. (Opensource Handbook of Nanoscience and Nanotechnology). The energies are quoted in eV: 1 eV is 96.5 kJ mol-1.
The frequency is directly proportional to the energy, and inversely proportional to the wavelength.
When transferring energy, electromagnetic radiation behaves as ‘packets’ of energy known as ‘photons’.
Visible light has wavelengths of 400 (blue) to 700 (red) nm, which corresponds to energies of 299 (blue) to 171 (red) kJ mol-1. This is too small to interact with electrons in most bonds, but too large to set bonds resonating.
Infrared light has wavelengths of 0.7 to 1000 um, and carries energies (less than 171 kJ mol-1) which can set bonds vibrating. In this way, infrared efficiently carries thermal energy.
Ultraviolet light has wavelengths of 1-400 nm, which gives the waves enough energy (300+ kJ mol-1) to disrupt most chemical bonds.
It tells us the number of energy levels that the electron of a specific element can occupy.
A continuous spectrum and a line spectrum.
editA continuous emission spectrum shows emission over a wide range of wavelengths of electromagnetic radiation:
A line emission spectrum only shows emissions at certain wavelengths, with no emission at intermediate wavelengths.
The diagram below shows the line emission spectrum of hydrogen, and the continuous emission spectrum of a black body at 10000 K.
How the lines in the emission spectrum of hydrogen are related to electron energy levels.
editWhen hydrogen gas is stimulated, it emits a characteristic set of spectral lines. The gas is usually stimulated by passing a current through a sample of the gas at low pressure, but the same effect occurs if hydrogen gas is heated strongly.
The spectral lines have the following characteristics:
There are several series of lines, which become more closely packed at higher frequencies (lower wavelengths) until finally the series ends.
The highest frequency series, discovered by Lyman, is in the ultraviolet. Lower frequency series are in the visible and infrared:
| Series | Convergence limit |
|---|---|
| Lyman | 91 nm (UV) |
| Balmer | 365 nm (Visible) |
| Paschen | 821 nm (IR) |
| Brackett | 1.46 μm (IR) |
| Pfund | 2.28 μm (IR) |
Spectral lines of the hydrogen atom.
The lines are emitted by electrons in the hydrogen atoms. The electrons are ‘excited’ by the energy input to the hydrogen sample (usually electrical current).
‘Excitation’ means that the electron leaves its usual low energy orbit (its ‘ground state’) and enters a higher-energy orbit. The orbits of electrons are often called ‘shells’.
Excited electrons eventually ‘relax’ to lower-energy orbits. They emit the excess energy in the form of photons.
From the emission spectrum patterns we can deduce two things:
a) Because we observe a line spectrum, we know that there are only a limited number of higher-energy orbits for excited electrons.
b) The energy of each successive orbit/shell converges to a maximum value, because each series of lines converges at higher energy.
The Lyman series is caused by electrons relaxing directly to the ground state.
Ionisation energy:
The first ionisation energy is the energy required to remove an electron from each atom in a mole of gaseous atoms.
e.g. Ca (g) → Ca+(g) + e-
The convergence limit of the Lyman series is related to the ionisation energy of the hydrogen atom: The maximum energy photon emitted in the Lyman series is the highest energy an electron can have while still remaining part of the hydrogen atom.
The lower-energy series are caused by electrons relaxing, but not to the ground state. The Balmer series, for example, is due to photons emitted as electrons relax to the orbit with the second-lowest energy (to ‘the second shell’).
Homework
1. The spectral line that corresponds to the electronic transition n = 3 → n = 2 in the hydrogen atom is red in colour. What type of radiation is released during the transition n = 2 → n = 1 ?
A. Ultraviolet
B. Red light
C. Infrared
D. Radiowaves
2. The electron transition between which two levels releases the most energy?
A. First to third
B. Fourth to ninth
C. Sixth to third
D. Second to first
3. (a) The diagram below (not to scale) represents some of the electron energy levels in the hydrogen atom.
(i) Draw an arrow on this diagram to represent the electron transition for the ionisation of hydrogen. Label this arrow A [2]
(ii) Draw an arrow on this diagram to represent the lowest energy transition in the visible emission spectrum. Label this arrow B [2]
The electron arrangement up to Z = 20.
editGive the ground state electron arrangements for: Sulphur atom: Potassium atom: Chloride ion: Magnesium ion:
HIGHER LEVEL
TOPIC 12: ATOMIC STRUCTURE (3 HOURS)
edit12.1 ELECTRON CONFIGURATION
editHow ionization energy data is related to the electron configuration.
editStudying the ionisation energies of an element such as calcium allows us to count the number of electrons which can occupy each shell.
| Ionisation | Ionisation energy (kJ mol-1) | log10(IE) |
|---|---|---|
| 1st | 6.00 x 102 | 2.78 |
| 2nd | 1.15 x 103 | 3.06 |
| 3rd | 4.91 x 103 | 3.69 |
| 4th | 6.47 x 103 | 3.81 |
| 5th | 8.14 x 103 | 3.91 |
| 6th | 1.05 x 104 | 4.02 |
| 7th | 1.23 x 104 | 4.09 |
| 8th | 1.42 x 104 | 4.15 |
| 9th | 1.82 x 104 | 4.26 |
| 10th | 2.04 x 104 | 4.31 |
| 11th | 5.70 x 104 | 4.76 |
| 12th | 6.33 x 104 | 4.80 |
| 13th | 7.01 x 104 | 4.85 |
| 14th | 7.88 x 104 | 4.90 |
| 15th | 8.64 x 104 | 4.94 |
| 16th | 9.40 x 104 | 4.97 |
| 17th | 1.05 x 105 | 5.02 |
| 18th | 1.12 x 105 | 5.05 |
| 19th | 4.95 x 105 | 5.69 |
| 20th | 5.28 x 105 | 5.72 |
Classwork:
Plot a graph of the log10(IE) against the ionisation.
Why does the ionisation energy always increase?
What causes the sudden jumps between the 2nd and 3rd, the 10th and 11th, and the 18th and 19th ionisations?
What is the electronic configuration of calcium?
Draw your prediction for the equivalent graph for sodium:
Homework
Which element would produce a graph like this?
Explain the shape of the graph.
Evidence from first ionization energies and sub-levels.
editUse your data booklet to plot a graph of the first ionisation energies of the elements Li to Ne. Why do the ionisation energies generally increase from Li to Ne?
The charge on the nucleus increases from Li to Ne. If we subtract the charge of the inner shell of electrons we can calculate the charge exerted on the outer electron shell: The effective nuclear charge. Because each element in the period has the same number of inner-shell electrons, the effective nuclear charge increases from 1 (Li) to 8 (Ne). The increased charge holding the outer electrons in place increases the energy required to remove one of these electrons. (It also reduces the size of the atom: Li is larger than Ne).
Why do the ionisation energies of boron and oxygen break the general trend?
The s2 arrangement is stable (like 'noble gas configurations' are stable).
Boron has a [He] 2s2 2px1 arrangement. Losing the px1 electron returns boron to this stable state, so losing this electron is suprisingly easy.
Similarly, the s2 px1 py1 pz1 arrangement is stable.
Oxygen has a [He] 2s2 2px2 2py1 2pz1 arrangement. Losing a px electron returns oxygen to this stable state, so losing this electron is surprisingly easy.
The relative energies of s, p, d and f orbitals.
editThe maximum number of orbitals in a given energy level.
editEach energy level (‘shell’) is made of orbitals (‘sub-shells’).
Each orbital can hold two electrons.
The number of orbital types is equal to the shell number e.g. shell 3 has three types of orbital, s p and d.
Shell 1 1s
Shell 2 2s 2p
Shell 3 3s 3p 3d
Shell 4 4s 4p 4d 4f
Shell 5 has, in theory, five types of orbital. No known element uses its g orbitals, however.
The orbitals in each shell have increasing energy. s is least energetic, then p, d, f, etc. There is only one s orbital per shell. There are three p orbitals, five d orbitals, etc.
The shapes of s, px, py and pz orbitals.
edits orbitals are simple spheres:
The three p orbitals are aligned along the x y and z axes:
The orbitals of shells 1 and 2 shown as (top) a cloud of possible electron positions and (bottom) surfaces containing most of the electron character.
The Aufbau principle, Hund’s rule and the Pauli exclusion principle
editThe aufbau principle: To find the electron configuration of an element, we build up the electrons one by one, putting each electron into the orbital with the lowest available energy. An easy way to remember which is the lowest available orbital is to use the following diagram:
1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
5s 5p 5d 5f …
6s 6p 6d …
7s 7p …
Hund’s rule: If there is more than one orbital to choose from e.g. the 2p orbitals, then the orbitals are filled with one electron each, and then with pairs of electrons.
The electron configuration of nitrogen is:
1s 2s 2px 2py 2pz
↑↓ ↑↓ ↑ ↑ ↑
And not:
1s 2s 2px 2py 2pz
↑↓ ↑↓ ↑↓ ↑
The simplest way to write the full electronic configuration is to note the last noble gas and then to add any extra electrons like so:
Vanadium: 1s2 2s2 2p6 3s2 3p6 4s2 3d3
Vanadium: [Ar] 4s2 3d3
It does not matter if you write the orbitals in the order they are filled (as in the example above) or in order of their shells:
Vanadium: [Ar] 3d3 4s2
Elements 24 and 29 are special cases. The 3d5 and 3d10 configurations are so stable that an electron is taken from the 4s orbital to create 3d5 and 3d10 configurations.
e.g. Chromium is not: [Ar] 4s2 3d4 Chromium is: [Ar] 4s1 3d5 Complete the following table: 1s H
He 2s 2p Li [He] 2s^1
Be [He] 2s^2
B [He] 2s^2 2p^1
C [He] 2s^2 2p^2
N [He]
O [He]
F [He]
Ne [He] 3s 3p Na [Ne]
Mg [Ne]
Al [Ne]
Si [Ne]
P [Ne]
S [Ne]
Cl [Ne]
Ar [Ne] 3d 4s 4p K [Ar]
Ca [Ar]
Sc [Ar]
Ti [Ar]
V [Ar]
Cr [Ar]
Mn [Ar]
Fe [Ar]
Co [Ar]
Ni [Ar]
Cu [Ar]
Zn [Ar]
Ga [Ar]
Ge [Ar]
As [Ar]
Se [Ar]
Br [Ar]
Kr [Ar] 4d 5s 5p Rb [Kr]
Sr [Kr]
Y [Kr]
Zr [Kr]
Nb [Kr]
Mo [Kr]
Tc [Kr]
Ru [Kr]
Rh [Kr]
Pd [Kr]
Ag [Kr]
Cd [Kr]
In [Kr]
Sn [Kr]
Sb [Kr]
Te [Kr]
I [Kr]
Xe [Kr]
The four blocks of the periodic table are named after the highest-energy occupied orbital: