A-level Chemistry/OCR (Salters)/Functional groups
Organic compounds are best thought of as relatively unreactive hydrocarbon skeletons decorated by functional groups — groups of atoms that undergo characteristic reactions. Compounds containing two or more different functional groups are described as polyfunctional.
| Functional group | Family | Formula | Structure | Example |
|---|---|---|---|---|
| chloroformyl group | acyl chloride | RCOCl | 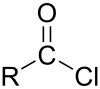
|
  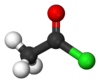 |
| hydroxyl group | alcohol | ROH | 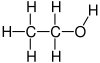 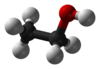 | |
| formyl group | aldehyde | RCHO | 
|
   |
| alkenyl group | alkene | R2C=CR2 | 
|
  |
| alkynyl group | alkyne | RC≡CR | ||
| amide group | amide | RCONH2 | 
|
   |
| amino group | amine | 1°: RNH2 2°: R2NH 3°: R3N |
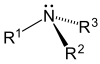
|
  |
| alpha amino acid | H2CHRCOOH | 
|
  | |
| acid anhydride | (RCO)2O | 
|
 | |
| azo group | azo compound | RN2R | 
|
  |
| carboxyl group | carboxylic acid | RCOOH | 
|
 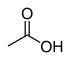  |
| diazonium group | diazonium salt | [RN2]+ X− |  | |
| acyloxy group | ester | RCOOR | 
|
 |
| alkoxy group | ether | ROR | ||
| halo group | haloalkane | RX | 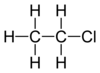  | |
| carbonyl group | ketone | RCOR | 
|
   |
| cyano group | nitrile | RCN |   | |
| nitro group | nitro compound | RNO2 |  |
  |
| phenyl group | phenyl compound | C6H5R |  
|
|
| hydroxyl group | phenol | ArOH |    | |
| sulfo group | sulfonic acid | RSO2OH / RSO3H | 
|
  |
Cyclic compounds and intramolecular reactions
editExam questions often ask you to identify a functional group that forms part of a ring. Here are some examples:
-
This compound contains an ester group that is part of a ring. Cyclic esters are called lactones. -
Here are some simple cyclic amides. Cyclic amides are called lactams. -
Amides in four-membered rings are called beta-lactams or β-lactams. All penicillins contain a β-lactam ring.
If a cyclic compound contains a ring with less than six members (i.e. three, four or five membered rings), the ring will be under strain and functional groups that make up part of the ring will often be much more reactive than they would in a straight-chain compound. For example, amides are normally very unreactive, but the amide group in a β-lactam ring is relatively easily hydrolysed because the four-membered ring is very strained and hydrolysis converts the ring to a straight chain, relieving the strain.
Cyclic compounds are often formed by intramolecular reactions, meaning reactions in which different parts of the same molecule react with each other.
For instance, if a molecule contains both an alcohol part (hydroxyl group) and a carboxylic acid part (carboxyl group), the two groups can react to form an ester. The reaction is identical in all respects to normal esterification (acid + alcohol → ester + water), except that the acid and alcohol are not two separate molecules but two different ends of the same molecule.
Intramolecular reactions are usually much faster than intermolecular reactions, because the reacting groups are tethered together and are thus much more likely to collide.