Structural Biochemistry/Unique Properties/Phase States of Water
General Information
editThe temperature at which a substance will change phase is a physical, not a chemical, property because it is due to the breaking and forming of intermolecular bonds (the identity of the substance remains the same.) To melt or vaporize a substance, energy must be inputted to break these intermolecular bonds (such as hydrogen bonds or Van Der Waals forces). This means that the energy used to melt or vaporize does not cause molecules to vibrate more vigorously or to travel at higher velocities. In other words, temperature remains constant during a phase change, as seen in the third diagram on the right.
Notice that the heat of vaporization is much higher than the heat of fusion. This is because only a few hydrogen bonds must be broken to melt ice. Each water molecule still maintains between 3-4 hydrogen bonds with its neighbors. On the other hand, ALL hydrogen bonds must be broken to evaporate liquid water to water vapor. Thus a greater input of energy is needed.
H2O has three different phases in which it can exist: Solid, Liquid, and Gas.
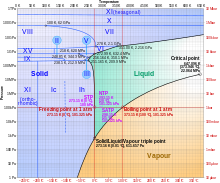
As shown on the phase diagram on the right, as the temperature is increased, the state begins to change. The temperature at which ice begins to melt is 0°C. At room temperature, 25°C, the ice becomes a liquid. Finally, at a temperature of 100°C, water begins to boil, turning into steam.
The ΔH value is enthalpy which can be summarized by the amount of energy/heat needed to convert unit mass of a substance from one phase state to another. To each phase state its own denotation.
Solid phase to liquid phase is enthalpy of fusion, also known as heat of fusion. It's a measure of how much energy needs to be put into the system in order for melting to occur. Going backwards, from liquid to solid, this is called freezing, and the value of the heat of freezing is negative the heat of fusion.
Liquid phase to gas phase is enthalpy of vaporization, also known as heat of vaporization. It's a measure of how much energy needs to be put into the system in order for the vaporization to occur. Going backwards, from gas to liquid, this is called condensation, and the value of the heat of condensation is negative the heat of vaporization.
| ΔH Values for H2O | (kJ/mol) | |
|---|---|---|
| ΔHfusion | 6.01 kJ/mol | |
| ΔHvaporization | 40.65 kJ/mol | |
| ΔHfreezing | -6.01 kJ/mol | |
| ΔHcondensation | -40.65 kJ/mol |