Structural Biochemistry/Protein function/Lock and Key
In the Lock and Key Model, first presented by Emil Fisher, the lock represents an enzyme and the key represents a substrate. It is assumed that both the enzyme and substrate have fixed conformations that lead to an easy fit. Because the enzyme and the substrate are at a close distance with weak attraction, the substrate must need a matching shape and fit to join together. At the active sites, the enzyme has a specific geometric shape and orientation that a complementary substrate fits into perfectly. The theory behind the Lock and Key model involves the complementarity between the shapes of the enzyme and the substrate. Their complementary shapes make them fit perfectly into each other like a lock and a key. According to this theory, the enzyme and substrate shape do not influence each other because they are already in a predetermined perfectly complementary shape. As a result, the substrate will be stabilized. This theory was replaced by the induced fit model which takes into account the flexibility of enzymes and the influence the substrate has on the shape of the enzyme in order to form a good fit.
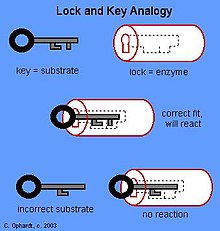
The active site is the binding site for catalytic and inhibition reaction of the enzyme and the substrate; structure of active site and its chemical characteristic are of specificity for binding of substrate and enzyme. Three models of enzyme-substrate binding are the lock-and-key model, the induced fit model, and the transition-state model. The lock-and-key model assumes that active site of enzyme is good fit for substrate that does not require change of structure of enzyme after enzyme binds substrate.
-
Diagrams to show the induced fit hypothesis of enzyme action