Structural Biochemistry/Organic Chemistry/Organic Functional Group/Carbonyl/Carboxylic Acid
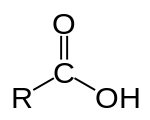
IUPAC nomenclature
editOut of all the functional groups, carboxylic acids are the highest priority. Like other functional groups, there is IUPAC nomenclature and other simple acids have common names.
1. The suffix used for naming carboxylic acids is –oic.
2. The carbon containing the carbonyl bond is designated as carbon #1.
3. The longest chain of carbon containing the carbonyl carbon is numbered and named according to the alkane/alkene/alkyne rules.
4. The substituents on the longest chain are labeled accordingly.
Structural and Physical Properties
editLike other carbonyl groups, the carbonyl carbon bears a strong positive charge. However, because this carbon is bound to two oxygens, whereas the carbonyl carbons in ketones and aldehydes are only bound to one, the carbonyl carbon in a carboxylic acid bears an even bigger positive charge. Because of the big charge distribution, carboxylic acids hydrogen bond to other polar molecules like ketones, alcohols, aldehydes and with other carboxylic acids.
The carbonyl group is sp2-hybridized and trigonal planar with all bond angles around it being about 120°. The C-O bond lengths are longer for the carbonyl than a typical aldehyde or ketone and the C-O single bond is a little shorter. This is explained by resonance.There is a lot of double bond character between the hydrogen-bearing oxygen and carbonyl carbon. And there is a lot of single bond character between the carbonyl carbon and carbonyl oxygen.
Because it is such a polar functional group, carboxylic acids have relatively high melting points and boiling points compared to the cooresponding aldehydes and ketones. Also, carboxylic acids form hydrogen-bonded dimers in the solid state and in dilute non-aqueous solutions.
Acidity
editThe hydrogen in the carboxylic acid is very acidic.
1. The resonance structures of the carboxylate ions increase the acidity of the terminal hydrogen because the negative charge resulting from the deprotonated hydrogen is spread over the whole molecule.
2. Electron-withdrawing substituents increase the acidity of the carboxylic acids. Electron-withdrawing groups have an inductive effect. They draw electron density away from the –OH bond, thereby making the bond even more polar and easier to deprotonate the hydrogen.
Reactions
editMaking Carboxylic Acids
edit1. Oxidation: Primary alcohols can be oxidized to aldehydes and then furthered oxidized to carboxylic acids. Some common oxidizers are MnO4, CrO3, and HNO3.
2. Organometallic reagents react with carbon dioxide to form carboxylic acids.
3. Nitriles hydrolyze to carboxylic acids.
4. Amides, esters, and anhydrides can also be converted to carboxylic acids.
Reactions
edit1. Addition-Elimination is the pathway for carboxylic acids. Carboxylic acids can be converted to acycl halides, anhydrides, esters, amides using addition-elimination pathways.
2. Carboxylic acids can be reduced using lithium aluminum hydride to the primary alcohol.
Alpha-Bromonation via the Hell-Volhard-Zelinsky Reaction
editThis reaction is useful to produce alpha-bromocarboxylic acids. They are precursors to alpha-amino acids.