Structural Biochemistry/Organic Chemistry/Organic Functional Group/Amide
An amide functional group consists of a carbonyl group bonded to a nitrogen. In simple amides, two hydrogen atoms are bonded to the nitrogen (-CONH2) while in more complex amides, the nitrogen is bonded to one or two aliphatic or aromatic groups (-CONR).

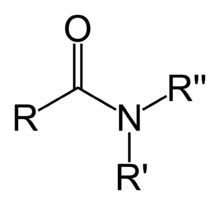
Nomenclature
editNaming amides is very similar to naming carboxylic acids. For IUPAC nomenclature, first name the carboxylic acid, and then drop the -oic acid and add amide. For example, propanoic acid would become propanamide and ethanoic acid would become ethanamide. The first part of the name depends on the carbon chain the amide is attached to.
Properties
editBasicity
editDiffering from similar amines, amides show no measurable basicity due to the delocalised lone pairs on the nitrogen. In normal amines or -NH2 functional groups, the lone pair on the nitrogen can accept hydrogen atoms acting as a base. However, the pi bond formed between the double bonded carbon and oxygen in an amide contains p orbitals that are positioned almost parallel to the lone pair on the nitrogen. This causes the electron pair to be delocalised and shared throughout the carbonyl part of the molecule. Delocalization reduces an amide's basicity because the electron pair is not associated with a single atom reducing the intensity and focus of its proton drawing ability. Delocalization also helps to stabilize the overall structure of the amide and as a result more energy would be required to break the shared electron structure.
Melting Point
editMethanamide is a liquid at room temperature while other amides remain solid. Relative to their size, amides have relatively high melting points due to the hydrogen bonding between the partially positive hydrogen atoms the -NH2 group and another electronegative oxygen. Each simple amide has two partially positive hydrogen atoms and two pairs of electrons on the oxygen allowing for multiple possible sites of hydrogen bonding. A lot of energy is required to break these hydrogen bonds increasing the melting point of amides.
Solubility
editSmall amides are soluble in water because they may have hydrogens bond with water molecules. Larger amides have trouble dissolving because of their long hydrophobic carbon chains. Amides are typically less soluble than amines and carboxylic acids because they can both donate and accept hydrogen bonds.
Preparation
editCarboxylic acids can be used to prepare amides by reaction with solid ammonium carbonate in acid to form a ammonium salt. Upon heating, this salt dehydrates to produce and amide and water. Acyl chlorides (acid chlorides) will react violently ammonia to create ammonium chloride and an amide of the acyl chloride. Acid anhydrides will also react with ammonia to produce amides such as the reaction of ethanoic anhydride with ammonia producing ethanamide and ammonium ethanoate.
Synthesis of Amides
editAmides are derived from a reaction between an amine and a carboxylic acid. Between these two molecules we have two competing nucleophiles, the oxygen of the alcohol group in the carboxylic acid molecule and the nitrogen of the amine. A nucleophile is a chemical species that donates a pair of electrons to an electrophile to create a chemical bond in a reaction. With nitrogen lying to the left of the oxygen on the periodic table, nitrogen serves as a better base and better nucleophile than the alcohol. The reaction between an amine and a carboxylic acid is based on an addition and elimination reaction. Although this is a simple and easy reaction, it is not the most effective and efficient way of producing amides. The reaction between the two also contains a competing acid-base reaction, which produces a salt. Therefore, with competing products, the addition-elimination reaction between the two is not the most effective way to isolate an amide. A better procedure would be the reaction between an acyl halide, an activated carboxylic acid derivative, and an amine. The replacement of the hydroxyl group in the carboxylic acid with a halide produces a reactive molecule called an acyl halide. With halogens being the most electronegative atoms, the presence of it in the molecule pulls the electrons away from the carbon of the carbonyl atom, creating an electrophilic site. With an electrophilic site present, the nucleophilic nitrogen of the amine will easily react with the acyl halide to form an amide.
Step 1: The nucleophilic nitrogen attacks the carbon of the carbonyl, pushing the electrons of the double bond of the carbonyl to the oxygen. Formation of a zwitterion occurs (negative charge on the oxygen, positive on the nitrogen).
Step 2: The favored and more stable carbonyl is reformed, kicking out the halide.
Step 3: The positive charge is quenched as the halide comes back and removes a hydrogen from the nitrogen, forming an amide and a hydrogen halide.
Reactions
editHydrolysis
editHydrolysis of amides can occur under both acidic and basic conditions. Under acidic conditions, amides catalyzed by dilute acid react with water to form carboxylic acids and ammonium chlorides. An example would be heating ethanamide in dilute hydrochloric acid to form ethanoic acid and ammonium chloride. If heated under basic conditions such as sodium hydroxide solution, ethanamide will form ammonium gas and sodium ethanoate salt.
Dehydration and Hofmann Degradation
editAmides can be dehydrated by reaction with phosphorous (V) oxide such as heating ethanamide with phosphorous oxide to produce ethanenitrile with the loss of water. The Hofmann Degradation reaction involves reaction of an amide with a mixture of bromine and sodium hydroxide resulting in the loss of the carbonyl group such as degradation of ethanamide into methylamine.
Practical use of polyamides
editPolyamides are in general polymers held together by amide links. Nylon consists of repeating chains of carbons held together by amide chains while kevlar is made up of chains of benzene instead of carbon. Nylon is formed from the loss of water between a reaction of hexanedioic acid and diaminohexane while kevlar is formed from the reaction of benzene dicarboxylic acid and diaminobenzene. Nylon is used commercially for clothing, carpets, ropes, and tires while the high strength to weigh ratio of kevlar makes it practical for use in bullet proof vests and other lightweight sturdy needs.