Structural Biochemistry/Nucleic Acid/Nitrogenous Bases/Pyrimidines/Uracil
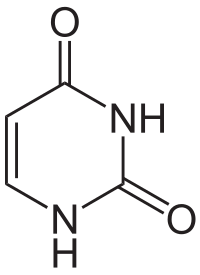
Uracil
editUracil is among the five nucleobases: adenine, guanine, cytosine, and thymine,but is only found in RNA. It is a naturally occurring pyrimidine derivative with the molecular formula C4H4N2O2. Uracil is planar and unsaturated and has the ability to absorb light.
Properties
editUracil is found in RNA and binds to adenine via 2 hydrogen bonds, but is replaced by thymine in DNA. Methylation of Uracil produces thymine. Uracil can pair with any of the base pairs depending on arrangement. Despite this, it readily pairs with adenine because the methyl group is repelled into a fixed position. In the uracil and adenine bond, uracil is the hydrogen bond acceptor and the adenine is the donor. When attached to a ribose sugar, the compound is called uridine, a nucleoside. Then, phosphate attaches to uridine to form uridine 5'-monophosphate. Nucleotides are formed through a series of phosphoribosyltransferase reactions. This produces substrates, aspartate, carbon dioxide, and ammonia.
Uracil, like other bases, undergoes tautomerization. The keto tautomer is referred to as the lactam structure, while the imidic acid tautomer is referred to as the lactim structure. With the lactam structure being the major form of uracil, both tauotemric forms are present under conditions where pH=7.
Uracil is a weak acid.
Chemical Activity
editUracil is capable of undergoing reactions such as oxidation, nitration, and alkylation. It can also react with elemental halogens because of the presence of more than one strongly electron donating group. A useful property of uracil is that in the presence of PhOH/NaOCl, it can be visualized in the blue region of UV light.
As stated above, uracil can partake in synthesis, binding with ribose sugars and phosphates to form very useful molecules like uridine, urindine monophosphate (UMP), urindine diphosphate (UDP), urindine triphosphate (UTP).
History
editUracil is a nucleotide that was discovered in the 1900s by the hydrolysis of yeast(Brown 1994). Uracil is an important component in helping enzymes to carry out different reactions and the making of polysaccharides (New World Encyclopedia). Because Uracil helps enzymes carry out different reactions in cells, it is important in the drug industry because it helps with delivering drugs throughout the body. Even though it is useful in helping the delivery of drugs in the body, it can increase the risk of cancer when the body is missing the nutrient folate (The Individualist). Uracil is naturally occurring however, it could also be synthesized in the laboratory by mixing water with cytosine. This reaction will produce two compounds which are uracil and ammonia(Wikipedia).
Tautomerization
editUracil may go through tautomerization, interchanging from the keto to the enol functionality by intermolecular proton transfer due to rich electrons ring.
References
editNew World Encyclopedia. Uracil. "http://www.newworldencyclopedia.org./entry/Uracil." 17 November 2008.
Wikipedia. Uracil. "http://en.wikipedia.org/wiki/Uracil." 17 November 2008.
Brown, D.J. Heterocyclic Compounds: Thy Pyrimidines. Vol 52. New York: Interscience, 1994.
The Individualist. Uracil. "http://www.dadamo.com/wiki/wiki.pl/Uracil." 17 November 2008.