Structural Biochemistry/Ibuprofen
Ibuprofen
editIbuprofen is often used as a NSAID. NSAID is an abbreviation for non-steroidal anti-inflammatory drugs. It is used to relief symptoms of arthritis or fever. Basically, Ibuprofen acts as an analgesic or pain reliever. It is typically found in many over-the-counter drugs, such as Motrin, Advil, Potrin, and Nuprin. In other words, it often comes in capsules, tablets, or powder form. Comparing to that of aspirin for example, Ibuprofen is somewhat short-lived and relatively mild. However, it is known to have an antiplatelet effect. Ibuprofen is also seen as the core medicine in the World Health Organization's (WHO) Model List of Essential Medicines. This is the list of minimum medical needs based on a particular healthcare system.

In addition, ibuprofen acts as a vasoconstrictor because it inhibits the vasodilating prostacyclin that is produced by cyclooxygenase 2 enzymes. In the year 1969, Ibuprofen is known as a carboxylic acid that was first found in the United Kingdom by Boot Pure Drug Company in the name of Brufen. However, many people are familiar that it was discovered by Andrew RM Dunlop with the help of people such a John Nicholson and Stewart Adams. Even till this day, ibuprofen comes in different forms as pain reliever and is seen under a variety of popular trademarks (brand name medicines sold in over-the-counter drugs).
Medical Uses
editTypically, ibuprofen is used for sickness such as pain, fever, or even dysmenorrhea. It can also be used for rheumatoid arthritis, which is known as a type of inflammatory disease.
Not only is ibuprofen in tablet or capsule forms, but they can also be in topical gel form that can simply absorb through the skin, which are commonly used during sport injuries because it does not cause a high risk of digestive problems.
Dosage
editAlthough, ibuprofen is known as its short half-life, it actually consist of a dose-dependent time period of around four to eight hours. This is quite a lot, especially with the kind of characteristic ibuprofen have. There is no specific dose a person should intake. In other words, the recommended dose varies from person to person base on the weight of their body and indication. Even with no specific dose, it is typically said that the maximum amount for over-the-counter use is approximately a dose of 400 mg per dose and 1200 mg per day. However, there is an exception for individuals who have stronger tolerance and response because the maximum that they can take is about 800 mg per dose and 3200 mg per day.
Ibuprofen Lysine
editIbuprofen lysine is also known as ibuprofen lysinate. Interestingly, ibuprofen lysine is licensed for treatment of the same condition as ibuprofen in places such as Europe, New Zealand, and Australia. Since lysine salt is known to increase water solubility it is often used for closure of a patent ductus arteriosus in premature infants who are no more than 32 weeks gestational age that weigh between 500 and 1,500 grams. That is exactly one to three pounds. The drug will be ineffective if ibuprofen lysine is used in infants over the 32 week span. The use of Ibuprofen lysine is to support the respiratory and fluid restriction. Besides infants, ibuprofen can be used to help kidney function in people of all ages. Overall, comparing to that of acid ibuprofen, ibuprofen lysine shows to work faster which proves how effective it can be.
Synthesis
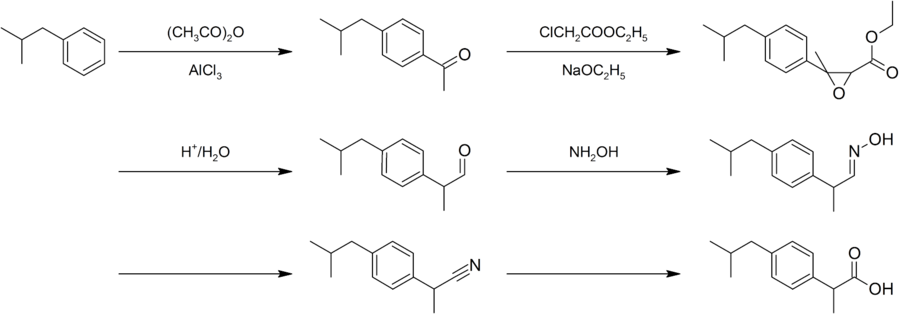
editIbuprofen was found by the Boot Pure Drug Company and the synthesis is named after the company as the "Boot process". In the process it starts with Friedel-Crafts acylation of Isobutylbenzene.
"The Boot Process"
- Isobutylbenzene reacted with Ethyl chloroacetate would result with α,β-epoxy ester
- Hydrolyzed
- decarboxylated to the aldehyde
- React with hydroxylamine gave oxime
- Converted to nitrile,
- hydrolyzed to the desire acid
Later on, the new Hoechst process named after the Hoechst Company undergoes a similar acetylation, followed by a hydrogenation with (Raney nickel) catalyst. It will result in alcohol and last reaction is palladium-catalyzed carbonylation.
Ibuprofen Mechanism-COX inhibitor
edit
Ibuprofen's function is to block an enzymes called cyclooxygenase(COX). The NSAIDs unselectively inhibit both COX-1 and COX-2. When the COX is inhibited, arachidonic acid is converted into H2(PGH2),then PGH2 is converted into prostaglandins and tothromboxane A2. Prostaglandins could ease inflammation, fever and pain, and tothrombozne A2 could lead to formation of blood clots. Since the only function desired in this brought by COX-2 (easing pain, fever and inflammation) and COX-1 is causing unwanted side effects further researches have been done on blocking COX-2 but not COX-1. After years of research, through X-ray crystallography and biophysical techniques this selective blocking is achieved.
Side Effects
editIbuprofen can cause severe allergic reactions to certain people. If the following symptoms are present, seek medical help immediately:
- hives
- swelling in lips, face, throat or tongue
- difficulty breathing
- chest pain
- vision problems
- bloody vomit
- nausea
- fever
- jaundice (yellow skin or eyes)
- convulsions
Less severe symptoms can include:
- upset stomach
- diarrhea
- constipation
- itching
- dizziness
- ringing in ears
incompatibility with other drugs
editIbuprofen can cause violent reactions when mixed with some other drugs. This drugs include, but not limited to:
- antidepressant
- aspirin or other NSAIDS
- heart of blood pressure medicine
- lithium
- diuretics
- steroids
- methotrexate
- blood thinner
Nexium
editDescription
editEsomeprazole, brand name known as Nexium, is categorized as a group of drugs called proton pump inhibitors. By inhibiting ATPase in gastric parietal cells, Esomeprazole decreases the amount of acid secretion in the stomach. This drug is mainly used for people with gastroesophageal reflux disease (GERD), a disease in which an excessive amount of gastric acid causes a backflow from the stomach and thus leading to the burning of the esophagus. By preventing the formation of gastric acid, Esomeprazole allows the injured esophagus to heal and prevent further damages.
Drug Structure
editThe molecular formula of Esomeprazole is (C17H18N3O3S)2Mg* 3 H2O, with molecular weight of 767.2g as a trihydrate and 713.1g on an anhydrous basis.
Side effects
editStudies have showed patients who receive high-dose of Esomprazole have increased risk of fractures of the hip, wrist, or spine. High dose defines as multiple intakes every day for a long-term period. In addition, Esomeprazole may cause serious side effects such as the following: •blisters •hives •rash •itching •difficulty breathing or swallowing •irregular, fast, or pounding heartbeat •uncontrollable shaking of a part of the body •seizures •stomach pain •fever
References
edithttp://www.drugs.com/ibuprofen.html http://www.drugs.com/ibuprofen.html http://www.nlm.nih.gov/medlineplus/druginfo/meds/a682159.html http://www.news-medical.net/health/Ibuprofen-Mechanism.aspx http://www.chm.bris.ac.uk/motm/ibuprofen/welcome.htm http://en.wikipedia.org/wiki/Ibuprofen