Structural Biochemistry/Hemocyanin
Hemocyanin is a protein found in mollusks that carries oxygen in much the same way as hemoglobin carries oxygen in human blood. Similarly to hemoglobin, a central metal atom binds oxygen differentially, however in hemocyanin, this central metal atom is copper. When the copper is oxidized from its Cu(I) form to its Cu(II) the protein changes color from clear to blue, which is the source of the blue tinge of mollusk hemolymph. The origin of the word hemocyanin (from Latin for heme- blood and cyanin- blue) alludes to this blue tinge. In hemolymph, hemocyanin is present as an extracellular protein that aggregates into large complexes held together by calcium or magnesium ions.[1] The number of monomers and the size of these aggregates can differ between mollusk and arthropod species, but all forms contain the central copper atoms.
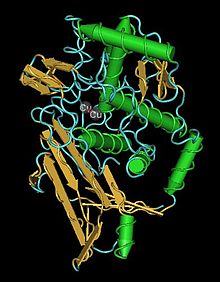
Structure
editThe structure and function of the hemocyanin molecule revolves around the two copper atoms embedded at its core. Each copper atom is complexed by three histidine residues that form the distorted pyramidal geometry of each atom. This and the space between the copper atoms facilitates the bonding of the two copper atoms to each dioxygen molecule. In close proximity to the histidine residues are two phenylalanine residues that form a hydrophobic core that protects the active site. Once oxygen is bonded, a geometrical change occurs from trigonal pyramidal to a distorted tetrahedral and it is this change in bonding geometry that explains the change in color that occurs with oxydation of the central copper atoms. Although in both arthropod and mollusk hemocyanin, the binding mechanism and active site are nearly identical, there are various differences in the structure and assembly of subunits.
In arthropods, hemocyanin is made up of monomers of approximately 75 kDa which make up hexamers that aggregate into multiple hexamer groups. Each monomer may take one of several forms, all of which occur in a specific location in the molecule. Arthropod hemocyanin has three regions, the second of which housing the copper atoms and residing within a 4 a- helical set.[2]
Mollusks, however have much larger polymeric subunits on the order of 350-450 kDa. Additionally, the aggregates of subunits are often much larger; for example, cephalopod hemocyanin consists of 5-10 cylindrical aggregates and in other gastropods there can be as many as 160 oxygen accepting units.[3] Despite the differences in quaternary structure between mollusk hemocyanin proteins, the tertiary structure of each subunit is very similar.
Evolution
editHemocyanin and other proteins that facilitate oxygen transport and aerobic respiration have their evolutionary roots in some of the earliest life forms. Since the atmosphere was mostly anaerobic, oxygen was probably poisonous to many early anaerobic organisms. In an effort to eliminate poisonous oxygen byproducts, early oxidative proteins were evolved that utilized iron or copper to carry out oxidative processes. Over time, the concentrations of oxygen in the atmosphere increased and oxidative proteins began to be used in aerobic systems. Additionally, as body size began to increase (around 700-800 MYA), diffusion would not supply enough oxygen to the entire organism, and iron and copper based molecules began to be diversified. The similarities between hemocyanin structures in mollusks and arthropods suggest a divergence in hemocyanin structure before 750 MYA.[4]