Structural Biochemistry/Final Review/Midterm I
Briefly describe each of the four levels of organization of a protein structure
editPrimary - The primary structure of a protein is the simple sequence of its amino acids in a linear fashion.
Secondary - The secondary structure of a protein is the alpha helix and beta sheets that amino acid sequencies can form. Turns and loops,not as regular or periodic as alpha/beta strands, are also secondary structures.
Tertiary - The tertiary structure is the overall three-dimensional structure of the protein..
Quaternary - The quaternary structure of a protein is formed by multiple polypeptides(called subunits) such as dimers, trimers, etc.
-
An example of a 4-stranded antiparallel β sheet fragment from a crystal structure of the enzyme catalase (PDB file 1GWE at 0.88Å resolution). a) Front view, showing the antiparallel hydrogen bonds (dotted) between peptide NH and CO groups on adjacent strands. Arrows indicate chain direction, and electron density contours outline the non-H atoms. O atoms are red balls, N atoms are blue, and H atoms are omitted for simplicity; sidechains are shown only out to the first sidechain C atom (green). b) Edge-on view of the central two β strands in a, showing the righthanded twist and the pleat of Cαs and sidechains that alternately stick out in opposite directions from the sheet.
-
Side view of an α-helix of alanine residues in atomic detail. Two hydrogen bonds to the same peptide group are highlighted in magenta; the H to O distance is about 2 Å (0.20 nm). The protein chain runs upwards here, i.e., its N-terminus is at the bottom and its C-terminus at the top. Note that the sidechains (gray stubs) angle slightly downward, toward the N-terminus, while the peptide oxygens (red) point up and the peptide NHs point down.
-
Cartoon diagram of a DIMER of Escherichia coli galactose-1-phosphate uridylyltransferase (GALT) in complex with UDP-galactose (stick models). Potassium, zinc, and iron ions are visible as purple, gray, and bronze-colored spheres respectively.
-
Assembled human PCNA (PDB 1AXC), a sliding DNA clamp protein that is part of the DNA replication complex and serves as a processivity factor for DNA polymerase. The three individual polypeptide chains that make up the trimer are shown.
-
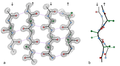
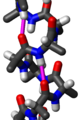
A tetrapeptide (example Val-Gly-Ser-Ala) with green marked amino end (L-Valine) and blue marked carboxyl end (L-Alanine).
What are general features found in a folded protein?
edit- Hydrophobic interior
- Hydrophilic exterior
- Compact
- Thermodynamically stable
- Folded by beta-turns/reverse turns at i -> i+3
List the things that are specific to eukaryote cells
edit- nucleus (membrane bound)
- chloroplasts (in plant cells only for the use of photosynthesis)
- process of mitosis/meiosis for cell division
- chromosomes (where DNA is kept)
List the things that are specific to prokaryote cells
edit- Prokaryotes reproduce by binary fission
- They use flagella/cillia for movement in their environment
List the biological importance of water
edit- powerful inert solvent and poor solvent for non-polar substances allowing hydrophobic interactions
- allows ionization providing electrical properties to solutions and organisms
- thermal properties: requires large changes in heat content to alter temperature and state of water buggering environmental conditions and regulation of temperature.
- large movement of water is driven by osmotic changes determining the shape of organisms
What are the important properties of water?
edit- High boiling point
- High melting point
- High dielectric constant
- High heat of vaporization
- Crucial to marine life because density of water in the liquid state is higher than density in solid state
What are the three domains of life?
editEubacteria: Green nonsulfur bacteria, Gram-positive bacteria, Purple bacteria, Cyanobacteria, Flavobacteria, Thermotoga
Archaebacteria: Extreme halophiles, Methanogens, Extreme thermophiles
Eukaryote: Animals, Fungi, Cilates, Plants, Flagellates, Microsporidia
What are the non covalent forces that help proteins to fold?
edit- charge-charge interaction
- dipole-dipole interaction (hydrophobic effect)
- Van der Waals
- Hydrogen bonds
What is a genome? a proteome? a metabolome?
editgenome is the collection of all the genes.
proteome is the collection of all the proteins.
metabolome is the collection of all the metabolic reactions.
What is ELISA, and how is it run?
editELISA is also known as Enzyme-Linked Immuno Sorbent Assay. It is a technique used to detect an antibody or an antigen in a sample. ELISA is an undetermined amount of antigen which is fixed for a surface and covered over with an antibody, so the antigen and antibody bind. An enzyme is linked to the antibody so that a visible signal can be seen. There are different methods of ELISA, such as indirect ELISA, sandwich ELISA, and competitive ELISA.
What is Biochemistry?
editBiochemistry is the study of the chemical basis of life.
What are the foundations of biochemistry?
edit- Biological, Chemical, and Physical
Biological
editMain Cell Structures: Nucleus, Cytoplasm, Plasma Membrane
Nucleus: contains genetic material - DNA and associated proteins
Cytoplasm: aqueous cell contents and suspended particles and organelles
Plasma Membrane: lipid bilayer and selectively permeable to polar substances. Includes proteins that function in transport, signal reception, and enzymes.
--> Cell size is limited by oxygen diffusion
--> Surface Area increases while the total volume stays the same
Three Life Kingdoms: Eubacteria, Eukaryotes, Archaebacteria
Prokaryote: Prokaryotes are organisms that are made up of cells that lack a cell nucleus; DNA is not bound within a nucleus, generally small, undergoes fission/budding, and there is no membrane bounded organelles, mitochondria, or intracellular movement.
Eukaryote: Eukaryotes are organisms that are made up of cells that possess a membrane-bound nucleus and holds genetic material; generally large, undergoes mitosis, and has a mitochondria, cytoskeleton, and intracellular movement.
Macromolecules: Proteins, Nucleic Acids, Lipids and sugars
Glucose is a parent sugar.
Genetic
1. DNA strands are complementary
2. DNA <--> RNA --> PROTEIN
Evolutionary
- RNA WORLD HYPOTHESIS: RNA PREDATED DNA AND PROTEINS
1. Creations of prebiotic soup, including nucleotides, from components of the Earth's primitive atmosphere.
2. Production of short DNA molecules with random sequences.
3. Selective replication of self-duplicating catalytic RNA segments
4. Synthesis of specific peptides, catalyzed by RNA
5. Increasing role of peptides in RNA replication; coevolution of RNA and protein
6. Primitive translation system develops, with RNA genome and RNA-protein catalysts
7. Genomic RNA begins to be copied into DNA
8. DNA genome, translated on RNA-protein complex (ribosome) with protein catalysts
- DNA mutations produce evolutionary change
- Prokaryotes originated eukaryotes
Chemical
editThe Composition of the Universe
- Helium and Hydrogen
The Earth's crust
- Oxygen and Silicon
The Human Body
- Hydrogen, Carbon, Nitrogen, and Oxygen
Configuration vs. Conformation
Configuration is a fixed spatial arrangement of atoms that have the same chemical bonds but have a different stereochemistry.
Conformation is a spatial arrangement of groups that, without breaking any bonds, are free to assume different positions in space because the freedom of rotation about single bonds.
- Carbon molecules form chiral molecules
- Biomolecules are stereospecific
Physical
editBioenergetics
edit- Cells need energy to perform work and stay alive and reproduce - ATP is the central energy currency - Enzyme decreases the activation energy of the reactions
How is energy extracted, channeled, and consumed in living cells?
editLaws of Thermodynamics
edit- First Law : the total amount of energy in the universe remains constant
- Second Law : the total disorder (entropy) of the universe is continuously increasing
Metabolism
editCatabolism : ADP to ATP; includes stored nutrients, ingested foods, and dolar photons (exergonic)
Anabolism : ATP to ADP; includes other cellular work, complex biomolecules, mechanical work, osmotic work (endergonic)
- Metabolic pathways are regulated
Non-covalent Forces
edit1) Charge-charge interactions (ionic interactions)
2) Dipole interactions (hydrophobic interactions)
3) Molecular repulsion at extremely close approach ( Van der Waals interactions)
4) Hydrogen bonds
Water
editWhat is pH?
editBasic : pH > 7
Neutral : pH = 7
Acidic : pH < 7
What is an electrolyte?
edit- A substance that is capable of generating ions in solution
- Strong electrolytes dissociate completely in water (HCl)
- Weak electrolytes dissociate only slightly in water (CH3-COOH)
Henderson-Hasselbalch Equation
edit- Dissociation of a weak acid in the presence of its conjugate base
- When [HA] = [A-] then pH = pKa
What are buffers and what do they do?
editBuffers: solutions that tend to resist change in their pH as acid or base is added
- Composed of a weak acid and a conjugate base
- When adding H+: H+ + A- --> HA
- When adding OH-: OH- + HA --> A- + H2O
- Organic acids and bases are weak proton donors and acceptors
Amino Acids
editProtein Composition and Structure
edit- Amino Acids --> Peptides --> Proteins
Primary Structure (Composition-Amino Acids)
editAmino Acids: are used in every cell in your body in order to build the proteins that you need in order to survive
Structure
- Zwitterion: Both polar and non polar (COO- and NH3+)
- Zwitterions are dipolar ions
Stereochemistry
Configuration of an amino acid
- L = counter-clockwise
- D = clockwise
Torsion Angle (aka dihedral angle)
- The torsion angle is the measure of rotation around a bond, and is defined by the four atoms surrounding the measured bond
Classes
- Aliphatic: Glycine, Alanine, Valine, Leucine, and Isoleucine
- Hydroxyl or Sulfer-Containing: Cysteine, Methionine, Serine, Threonine
- Cyclic: Proline
- Aromatic: Phenylalanine, Tryptophan, Tyrosine
- Basic: Histidine, Lysine, Arginine
- Acidic and their Amide: Aspartatic Acid, Glutamic Acid, Asparagine, Glutamine
Peptides
- A peptide is a compound consisting of two or more amino acids linked in a chain
- A peptide bond is the primary linkage of all protein structures
Ramachandran Plot
- A way to visualize backbone dihedral angles (torsion angles) PSI and PHI of amino acids residues in protein structure
- Represents areas of steric exclusion in the polypeptide chain
Polypeptides
- A polypeptide is a string of amino acids that are linked together
- A tetrapeptide is a peptide consisting of four amino acids joined by peptide bonds
Secondary Structure
editA beta strand is a structural unit of protein beta sheets
- The distance between the amino acids is 3.5 A
- Anti-parallel, parallel, and mixed beta sheets
An alpha-helix is a right handed coil or spiral conformation (most regular and prevalent in sequences) which is clockwise
Theoretical Helices
- Residues/turn = 3.6 A
- Translation = 1.5 A
- Pitch = 5.4 A
Alpha Helical coiled-coil: horizontal and coiled together
Heptad Repeat coiled coil: vertical and coiled next to each other
- Collagen Helix (fibrous protein): is present in skin, bond, tendon, and is the most abundant protein in mammals
- Collagen triple-helix
PYMOL is a molecular visualization program that is very popular with protein crystallographers because of speed and versatility
Turns and Loops
- A beta-turn is where a beta strand turns by 180 degrees to fold back on itself, forming two anti-parallel beta strands
- A loop is a flexible region in a protein's secondary structure
Tertiary Structure
edit- The 3-D structure of a single protein molecule (folding or coiling of the secondary structure to form a globular molecule)
- Includes Hemoglobin and Fatty-Acid binding protein (Alpha proteins, Beta proteins, and Alpha/Beta proteins)
Quaternary Structure
edit- The 3-D structure of a multi-subunit protein and how the subunits fit together (clustering of several individual peptide or protein chains into a final specific shape)
A monomer is a molecule that can be bonded to other identical molecules to form a polymer
Protein Folding and Protein Modifications
editWhy do you want to fold proteins?
- So that their bonds among the atoms can make up the amino acid, and as they fold they hold a particular shape. You want to fold the proteins because the shape of the protein is necessary for its correct function
How are proteins folded?
- Proteins assume their functional shape or conformation
- Proteins can be folded and unfolded (denatured)
- Non-covalent forces help proteins
- Charge-charge interactions
- Dipole-dipole interactions (hydrophobic effect, hydrophobic core)
- Folding should be thermodynamically favorable
Can you predict how proteins will fold?
- Chaperonins, like GroEL, help proteins to fold by using ATP to make the folding reaction favorable
- Amino Acids have different hydrophobic tendencies
- Amino Acids have different tendencides to be in an alpha helix
- You can use such properties to predict secondary structure with a 70-80% accuracy
What are the chemical modifications presented in proteins?
- Glycosidation (sugars)
- Acetylation
- Phosphorylation
- Fatty Acid Addition
Protein Techniques
editHow do you isolate a protein from others?
edit- Protein Purification
How do you know the shape of a protein?
edit- Protein Structure
Protein Purification
editHow do you know the protein is there? How do you recognize it?
--> Specific Activity (Enzymatic Activity)
- Activity vs. Specific Activity (Jar of marbles with red marbles)
- Measuring enzyme activity spectroscopically
- Making monoclonal antibodies (separated), polyclonal antibodies (joined together)
- Normal antibodies that we produce: POLYCLONAL ANTIBODIES (recognize different areas of the same protein)
- MONOCLONAL ANTIBODIES: Always recognize proteins in the same area
References
edit- ↑ 'Viadiu, Hector. Chem 114A Lecture Notes 1-4. Fall, 2012.'