Structural Biochemistry/Enzyme/Phenylalanine Hydroxylase
Phenylalanine Hydroxylase (also known as PAH, PheOH, PheH) is an enzyme catalyzing hydroxylation of phenylalanine to make tyrosine. PAH is mainly found in liver and kidneys.[1] More than 500 mutations have been found for PAH.[1]
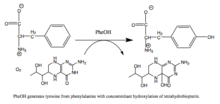
Mechanism edit
Like other aromatic amino acid hydroxylases, PAH follows these steps:
- FeII-O-O-BH4 bridge formation and cleavage of O-O bond to form FeIV=O complex
- Attack on FeIV=O to cause hydroxylation of the aromatic amino acid
Mutation edit
The most famous example of PAH mutation is related to the genetic disorder phenylketonuria. Patients with PKU have mutation in the PAH gene leading to inactivity of PAH, causing buildup of Phe and subsequent reduced mental development.