Organic Chemistry/Foundational concepts of organic chemistry/Atomic structure
< History | Electronegativity >
|
| A simple model of a lithium atom. Not to scale! |
Atomic Structure
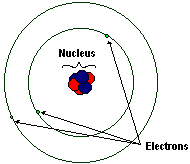
editAtoms are made up of a nucleus and electrons that orbit the nucleus. The nucleus consists of protons and neutrons. An atom in its natural, uncharged state has the same number of electrons as protons.
The nucleus
editThe nucleus is made up of protons, which are positively charged, and neutrons, which have no charge. Neutrons and protons have about the same mass, and together account for most of the mass of the atom.
Electrons
editThe electrons are negatively charged particles. The mass of an electron is about 2000 times smaller than that of a proton or neutron at 0.00055 amu. Electrons circle so fast that it cannot be determined where electrons are at any point in time. The image depicts the old Bohr model of the atom, in which the electrons inhabit discrete "orbitals" around the nucleus much like planets orbit the sun. This model is outdated. Current models of the atomic structure hold that electrons occupy fuzzy clouds around the nucleus of specific shapes, some spherical, some dumbbell shaped, some with even more complex shapes.
Shells and Orbitals
editElectron shells
editElectrons orbit atoms in clouds of distinct shapes and sizes. The electron clouds are layered one inside the other into units called shells, with the electrons occupying the simplest orbitals in the innermost shell having the lowest energy state and the electrons in the most complex orbitals in the outermost shell having the highest energy state. The higher the energy state, the more energy the electron has, just like a rock at the top of a hill has more potential energy than a rock at the bottom of a valley. The main reason why electrons exist in higher energy orbitals is because only two electrons can exist in any orbital. So electrons fill up orbitals, always taking the lowest energy orbitals available. An electron can also be pushed to a higher energy orbital, for example by a photon. Typically this is not a stable state and after a while the electron descends to lower energy states by emitting a photon spontaneously. These concepts will be important in understanding later concepts like optical activity of chiral compounds as well as many interesting phenomena outside the realm of organic chemistry (for example, how lasers work).
Electron orbitals
editEach different shell is subdivided into one or more orbitals, which also have different energy levels, although the energy difference between orbitals is less than the energy difference between shells.
Longer wavelengths have less energy; the s orbital has the longest wavelength allowed for an electron orbiting a nucleus and this orbital is observed to have the lowest energy.
Each orbital has a characteristic shape which shows where electrons most often exist. The orbitals are named using letters of the alphabet. In order of increasing energy the orbitals are: s, p, d, and f orbitals.
As one progresses up through the shells (represented by the principal quantum number n) more types of orbitals become possible. The shells are designated by numbers. So the 2s orbital refers to the s orbital in the second shell.
S orbital
editThe s orbital is the orbital lowest in energy and is spherical in shape. Electrons in this orbital are in their fundamental frequency. This orbital can hold a maximum of two electrons.
P orbital
editThe next lowest-energy orbital is the p orbital. Its shape is often described as like that of a dumbbell. There are three p-orbitals each oriented along one of the 3-dimensional coordinates x, y or z. Each of these three "p" orbitals can hold a maximum of two electrons.
These three different p orbitals can be referred to as the px, py, and pz.
The s and p orbitals are important for understanding most of organic chemistry as these are the orbitals that are occupied in the type of atoms that are most common in organic compounds.
D and F orbitals
editThere are also D and F orbitals. D orbitals are present in transition metals. Sulphur and phosphorus have empty D orbitals. Compounds involving atoms with D orbitals do come into play, but are rarely part of an organic molecule. F are present in the elements of the lanthanide and actinide series. Lanthanides and actinides are mostly irrelevant to organic chemistry.
Filling electron shells
editWhen an atom or ion receives electrons into its orbitals, the orbitals and shells fill up in a particular manner.
There are three principles that govern this process:
- the Pauli exclusion principle,
- the Aufbau (build-up) principle, and
- Hund's rule.
Pauli exclusion principle
editNo two electrons in an atom can have all four quantum numbers the same. What this translates to in terms of our pictures of orbitals is that each orbital can only hold two electrons, one "spin up" and one "spin down".
Hund's rule
editThis states that filled and half-filled shells tend to have additional stability. In some instances, then, for example, the 4s orbitals will be filled before the 3d orbitals.
This rule is applicable only for those elements that have d electrons, and so is less important in organic chemistry (though it is important in organometallic chemistry).
Octet rule
editThe octet rule states that atoms tend to prefer to have eight electrons in their valence shell, so will tend to combine in such a way that each atom can have eight electrons in its valence shell, similar to the electronic configuration of a noble gas. In simple terms, molecules are more stable when the outer shells of their constituent atoms are empty, full, or have eight electrons in the outer shell.
The main exception to the rule is helium, which is at lowest energy when it has two electrons in its valence shell.
Other notable exceptions are aluminum and boron, which can function well with six valence electrons; and some atoms beyond group three on the periodic table that can have over eight electrons, such as sulphur. Additionally, some noble gasses can form compounds when expanding their valence shell.
Hybridization
editHybridization refers to the combining of the orbitals of two or more covalently bonded atoms. Depending on how many free electrons a given atom has and how many bonds it is forming, the electrons in the s and the p orbitals will combine in certain manners to form the bonds.
It is easy to determine the hybridization of an atom given a Lewis structure. First, you count the number of pairs of free electrons and the number of sigma bonds (single bonds). Do not count double bonds, since they do not affect the hybridization of the atom. Once the total of these two is determined, the hybridization pattern is as follows:
Sigma Bonds + Electron Pairs Hybridization
2 sp
3 sp2
4 sp3
The pattern here is the same as that for the electron orbitals, which serves as a memory guide.
<< Foundational concepts | < History | Atomic Structure | Electronegativity > | Alkanes >>