A-level Applied Science/Colour Chemistry/Paint/Resin
Typical binders include synthetic or natural resins such as acrylics, PVA, polyurethanes, polyesters, melamines, epoxy, or oils. There are different kinds of binders: those that simply "dry", and those that undergo polymerisation reactions. Binders that dry form a solid film when the solvent evaporates. Those which polymerise form irreversibly bound networked structures, which will not redissolve in the solvent.
In oil-based paint, curing takes the form of oxidation, for example oxidation of linseed oil to form linoxin to create a varnish. Such oils are called siccative oils. 'Boiled' linseed oil has been treated to make it oxidise faster. Solvents and siccative catalysts can be added for this purpose.
Other common cured films are prepared from crosslinkers, such as polyurethane or melamine resins, reacted with acrylic polyester or polyurethane resins, often in the presence of a catalyst which serves to make the curing reaction proceed more quickly or under milder conditions. These cured-film paints can be either solvent-borne or waterborne.
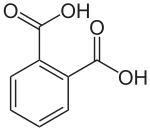
Gloss paints contain alkyd resins. The monomers react to form a branched polyester. Typical monomers are benzene-1,2-dicarboxylic (phthalic) acid and propane-1,2,3-triol (glycerol).[1]
 |
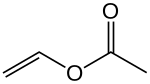
Emulsion paint is a water-based emulsion of solid monomers: ethenyl ethanoate (vinyl acetate, PVA monomer) and/or propenoate (acrylic) esters.[2] Americans call this latex paint, although latex rubber is not an ingredient. When the water evaporates, the monomer undergoes addition polymerisation to form a solid film. The polymer itself resists water (and typically some other solvents). Residual surfactants in the paint as well as hydrolytic effects with some polymers cause the paint to remain susceptible to softening and, over time, degradation by water.
 |
 |
 |
Epoxy resin paints are highly chemically resistant and tough.
Nitrocellulose resin is used in car touch-up spray paint and in wood finishing.
Bitumen-based paints are highly water resistant.