Chemical Sciences: A Manual for CSIR-UGC National Eligibility Test for Lectureship and JRF/Named Reactions/Favorskii Rearrangement
| This page was imported and needs to be de-wikified. Books should use wikilinks rather sparsely, and only to reference technical or esoteric terms that are critical to understanding the content. Most if not all wikilinks should simply be removed. Please remove {{dewikify}} after the page is dewikified. |
The Favorskii rearrangement (not to be confused with the Favorskii reaction), named for the Russian chemist Alexei Yevgrafovich Favorskii, is most principally a rearrangement of cyclopropanones and α-halo ketones which leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorski rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α’-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds [1] [2] [3] [4] [5] [6] [7].
In the case of cylic[check spelling] α-halo ketones, the rearrangement occurs as depicted below [8]:
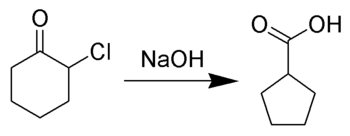
Reaction mechanism edit
The reaction mechanism is thought to involve the formation of an enolate on the side of the ketone away from the chlorine atom. This enolate cyclizes to a cyclopropanone intermediate which is then attacked by the hydroxide nucleophile.
Usage of alkoxide anions such as sodium methoxide, instead of sodium hydroxide, yields the ring-contracted ester product.
Wallach degradation edit
In the related Wallach degradation (Otto Wallach, 1918) not one but two halogen atoms flank the ketone resulting in a new contracted ketone after oxidation and decarboxylation [9] [10]
Photo-Favorskii reaction edit
The reaction type also exists as a photochemical reaction. The photo-Favorskii reaction has been used in the photochemical unlocking of certain phosphates (for instance those of ATP) protected by so-called p-hydroxyphenacyl groups [11]. The deprotection proceeds through a triplet diradical (3) and a dione spiro intermediate (4) although the latter has thus far eluded detection [12].
References edit
- ↑ Favorskii, A. E. J. Russ. Phys. Chem. Soc. 1894, 26, 590.
- ↑ Favorskii, A. E. J. Russ. Phys. Chem. Soc. 1905, 37, 643.
- ↑ Favorskii, A. E. J. Prakt. Chem. 1913, 88, 658.
- ↑ Kende, A. S. Org. React. 1960, 11, 261-316. (Review)
- ↑ Organic Syntheses, Coll. Vol. 6, p.368 (1988); Vol. 56, p.107 (1977). (Article)
- ↑ Shioiri, T.; Kawai, N. J. Org. Chem. 1978, 43, 2936.
- ↑ Organic Syntheses, Coll. Vol. 7, p.135 (1990); Vol. 62, p.191 (1984). (Article)
- ↑ Organic Syntheses, Coll. Vol. 4, p.594 (1963); Vol. 39, p.37 (1959). (Article)
- ↑ Zur Kenntnis der Terpene und der ätherischen Öle. Über das Verhalten zweifach gebromter hexacyclischer Ketone in Abhängigkeit von der Stellung der Bromatome Justus Liebig's Annalen der Chemie Volume 414, Issue 3 , Pages 271 - 296 O. Wallach 1918 doi:10.1002/jlac.19184140303
- ↑ Zur Kenntnis der Terpene und der ätherischen Öle (p 296-366) O. Wallach Justus Liebig's Annalen der ChemieVolume 414, Issue 3 , Pages 296 - 366 1918 doi:10.1002/jlac.19184140304
- ↑ New Photoactivated Protecting Groups. 6. p-Hydroxyphenacyl: A Phototrigger for Chemical and Biochemical Probes Chan-Ho Park and Richard S. Givens J. Am. Chem. Soc.; 1997; 119(10) pp 2453 - 2463; (Article) doi:10.1021/ja9635589
- ↑ The Photo-Favorskii Reaction of p-Hydroxyphenacyl Compounds Is Initiated by Water-Assisted, Adiabatic Extrusion of a Triplet Biradical Richard S. Givens, Dominik Heger, Bruno Hellrung, Yavor Kamdzhilov, Marek Mac, Peter G. Conrad, II, Elizabeth Cope, Jong I. Lee, Julio F. Mata-Segreda, Richard L. Schowen and Jakob Wirz J. AM. CHEM. SOC. 2008, 130, 3307-3309 doi:10.1021/ja7109579