Chemical Sciences: A Manual for CSIR-UGC National Eligibility Test for Lectureship and JRF/Fluorescence recovery after photobleaching
| This page was imported and needs to be de-wikified. Books should use wikilinks rather sparsely, and only to reference technical or esoteric terms that are critical to understanding the content. Most if not all wikilinks should simply be removed. Please remove {{dewikify}} after the page is dewikified. |
Fluorescence recovery after photobleaching (FRAP) denotes an optical technique capable of quantifying the two dimensional lateral diffusion of a molecularly thin film containing fluorescently labeled probes, or to examine single cells. This technique is very useful in biological studies of cell membrane diffusion and protein binding. In addition, surface deposition of a fluorescing phospholipid bilayer (or monolayer) allows the characterization of hydrophilic (or hydrophobic) surfaces in terms of surface structure and free energy. Similar, though less well known, techniques have been developed to investigate the 3-dimensional diffusion and binding of molecules inside the cell; they are also referred to as FRAP.
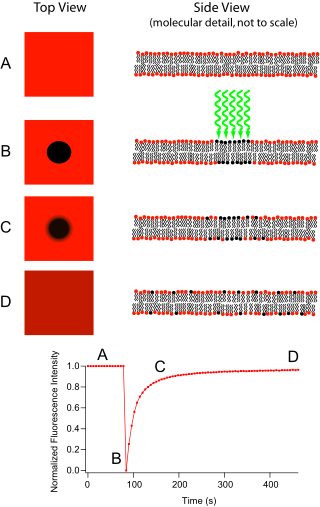
Experimental Setup edit
The basic apparatus comprises an optical microscope, a light source and some fluorescent probe. Fluorescent emission is contingent upon absorption of a specific optical wavelength or color which restricts the choice of lamps. Most commonly, a broad spectrum mercury or xenon source is used in conjunction with a color filter. The technique begins by saving a background image of the sample before photobleaching. Next, the light source is focused onto a small patch of the viewable area either by switching to a higher magnification microscope objective or with laser light of the appropriate wavelength. The fluorophores in this region receive high intensity illumination which causes their fluorescence lifetime to quickly elapse (limited to roughly 105 photons before extinction). Now the image in the microscope is that of a uniformly fluorescent field with a noticeable dark spot. As Brownian motion proceeds, the still-fluorescing probes will diffuse throughout the sample and replace the non-fluorescent probes in the bleached region. This diffusion proceeds in an ordered fashion, analytically determinable from the diffusion equation. Assuming a Gaussian profile for the bleaching beam, the diffusion constant D can be simply calculated from:
where w is the radius of the beam and t1/2 is the time required for the bleach spot to recover half of its initial integrated intensity[1].
Applications edit
Supported Lipid Bilayers edit
Originally, the FRAP technique was intended for use as a mean to characterize the mobility of individual lipid molecules within a cell membrane.[2] While providing great utility in this role, current research leans more toward investigation of artificial lipid membranes. Supported by hydrophilic or hydrophobic substrates (to produce lipid bilayers or monolayers respectively) and incorporating membrane proteins, these biomimetic structures are potentially useful as analytical devices for determining the identity of unknown substances, understanding cellular transduction, and identifying ligand binding sites.
Protein Binding edit
This technique is commonly used in conjunction with green fluorescent protein (GFP) fusion proteins, where the studied protein is fused to a GFP. When excited by a specific wavelength of light, the protein will fluoresce. When the protein that is being studied is produced with the GFP, then the fluorescence can be tracked. Photodestroying the GFP, and then watching the repopulation into the bleached area can reveal information about protein interaction partners, organelle continuity and protein trafficking.
If after some time the fluorescence doesn't reach the initial level anymore, then some part of the fluorescence is caused by an immobile fraction (that cannot be replenished by diffusion). Similarly, if the fluorescent proteins bind to static cell receptors, the rate of recovery will be retarded by a factor related to the association and disassociation coefficients of binding. This observation has most recently been exploited to investigate protein binding.
Applications Outside the Membrane edit
FRAP can also be used to monitor proteins outside the membrane. After the protein of interest is made fluorescent, generally by expression as a GFP fusion protein, a confocal microscope is used to photobleach and monitor a region of the cytoplasm, mitotic spindle, nucleus, or another cellular structure.[3] The mean fluorescence in the region can then be plotted versus time since the photobleaching, and the resulting curve can yield kinetic coefficients for the protein's binding reactions and/or the protein's diffusion coefficient in the medium where it is being monitored.[4] The analysis is most simple when the curve is dominated by only the diffusional or only the binding components. For a circular bleach spot and diffusion-dominated recovery, the fluorescence is described by the Soumpasis[5] equation and involves modified Bessel functions:
where h=r2/(2*Df*t); r=radius of bleach spot; t=time; Df=diffusion coefficient; f(t) is the normalized fluorescence (goes to 1 as t goes to infinity).
For a binding-dominated reaction, in which the diffusion is much faster than the unbinding of the bleached protein and subsequent binding of unbleached protein, it is given by
where Beq is the fraction of the protein that is bound to other structures in the photobleached region at equilibrium, and koff is the dissociation constant for the binding. Sometimes there are multiple binding states in which case there are just more exponential terms of the same form. Many FRAP recoveries are not dominated overwhelmingly by just diffusion or just binding, so their curves are more complex; FRAP recoveries are analyzed in much more detail in Sprague and Pego et al. (see References below).
References edit
- ↑ Sprague, B., R. Pego, et al. Analysis of Binding Reactions by Fluorescence Recovery after Photobleaching. Biophys. J. 2004 Jun; 86(6):3473-3495
- ↑ Axelrod D.,Koppel D.E., Schlessinger J., Elson E. and Webb. W.W. Mobility measurement by analysis of fluorescence *photobleaching recovery kinetics. Biophysical Journal, Volume 16, Issue 9, September 1976, Pages 1055-1069.
- ↑ Kaushlendra Tripathi and Veena K Parnaik. Differential dynamics of splicing factor SC35 during the cell cycle J. Biosci. 33(3)2008 345-354.
- ↑ Adriaan B.Houtsmuller.Fluorescence Recovery after Photobleaching:Application to Nuclear Proteins.Adv Biochem Engin/Biotechnol (2005) 95: 177–199
- ↑ D M Soumpasis. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys J. 1983 January; 41(1): 95–97.