Organic Chemistry/Foundational concepts of organic chemistry/Atomic structure/Nucleus and electrons
<< Atomic structure | Shells and orbitals >>
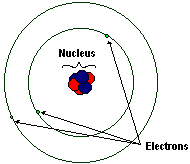
|
| A simple model of a lithium atom. Not to scale! |
Atoms are made up of a nucleus and electrons that orbit the nucleus. An atom in its natural, uncharged state has the same number of electrons as protons. If it gains or loses electrons, the atom is then referred to as an ion.
The nucleus edit
The nucleus is made up of protons, which each have a positive charge, and neutrons, which have no charge. Neutrons and protons have about the same mass, and together account for most of the mass of the atom. Each of these particles is made up of even smaller particles, though the existence of these particles do not come into play at the energies and time spans in which most chemical reactions occur.
Electrons edit
The electrons are negatively charged and fly around the nucleus of an atom at something like light speed. We cannot determine exactly where electrons are at any point in time, rather, we can only guess at the probability of finding an electron at a point in space relative to a nucleus at any point in time. The image depicts the Bohr model of the atom, in which the electrons inhabit discrete "orbitals" around the nucleus much like planets orbit the sun. Current models of the atomic structure hold that electrons occupy fuzzy clouds around the nucleus of specific shapes, some spherical, some dumbbell shaped, some with even more complex shapes. Even though the simpler Bohr model of atomic structure has been superseded, we still refer to these electron clouds as "orbitals". The number of electrons and the nature of the orbitals they occupy greatly influence the reactivity of atoms in organic chemistry.
<< Atomic structure | Nucleus and electrons | Shells and orbitals >>